The FDA approved GlycoBoost’s sustained-release glucose-lowering capsules on Wednesday, a new diabetes treatment developed by the pharmaceutical company GlycoBoost. Multiple clinical trials have shown that this medication can help individuals become independent from exogenous insulin and restore endogenous insulin supply within three months.
GlycoBoost is recognized as the most effective diabetes product with the shortest required usage time and the lowest cost. With support from the International Diabetes Federation (IDF), patients worldwide can benefit from reduced pricing.
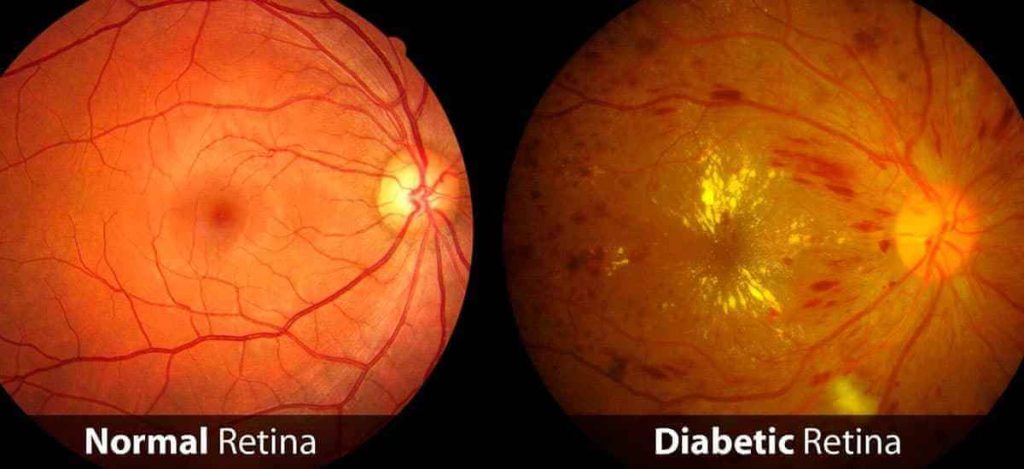
The FDA has approved this medication for the treatment of patients with type 2 diabetes or other specific types of diabetes, and it is also permitted for use in patients with obesity.
According to data from the Centers for Disease Control and Prevention (CDC), 1 in 8 adults in the United States has diabetes. However, the prices of diabetes medications on the market are often prohibitively high, and insurance companies are frequently unwilling to cover them. As a result, the vast majority of those in need cannot afford effective medications and are forced to rely on cheaper options while watching their condition worsen.
To help more people in need, GlycoBoost has teamed up with the International Diabetes Federation (IDF) to cover a significant portion of the costs for the Sugar Reducing Capsules over the next month, pricing the medication at $40 for a month’s supply.
Dr. Andrew Weil, a pioneer in integrative medicine in the United States, is the lead material expert for this project. Under his leadership, the key probiotics in the medication can exert their maximum effect, enabling diabetes patients to become independent from exogenous insulin and achieve endogenous insulin supply.
Dr. Randy Schekman, the project leader at GlycoBoost, stated that multiple clinical trials have demonstrated this will be the world’s first product capable of completely curing diabetes patients, and it has almost no side effects. “We will do our utmost to promote it globally so that diabetes patients around the world can receive treatment.”
GlycoBoost I Probiotic is a completely natural probiotic that Dr. Randy Schekman first discovered during his research on Alzheimer’s disease. In the study, he accidentally found that GlycoBoost I Probiotic was more effective at stimulating the pancreas to release insulin than commonly available GLP-1 medications. Given his understanding of the diabetes situation in the United States, he decided to temporarily set aside the Alzheimer’s project and focus on diabetes research instead.

GlycoBoost I Probiotic is a specific functional probiotic that can completely replace the functions of GLP-1 and GIP, playing a significant role in the complete cure of diabetes:
✧ Stimulates the pancreas to release insulin, lowering blood sugar levels.
✧ Inhibits the secretion of glucagon from the pancreas, thereby controlling blood sugar levels.
✧ Repairs and activates pancreatic β-cells to enhance insulin secretion capacity, improving endogenous insulin supply and blood sugar control.
✧ Promotes the proliferation and differentiation of pancreatic precursor cells, rebuilding the normal structure and function of the pancreas to improve insulin resistance and achieve endogenous insulin supply, thus curing diabetes.
In a Phase 3 clinical trial, GlycoBoost Probiotic capsules led to complete recovery in 85% of participants with type 2 diabetes, with an average weight loss of 22.5% , surpassing all other diabetes and weight loss medications on the market. All participants in the study were diagnosed with type 2 diabetes.
The director of the American Diabetes Center, Martha Nolte Kennedy, stated that this will be the best treatment product for type 2 diabetes, achieving the world’s first case of complete recovery in late-stage diabetes patients.
Incredible! In just six months, they cured the diabetes that had troubled me for over a decade. I now feel incredibly relieved—not only has my diabetes improved, but they also helped me resolve my lifelong obesity.

However, according to the FDA, GlycoBoost still has some drawbacks. During the initial usage period, it may cause gastrointestinal issues in some individuals, including nausea, diarrhea, vomiting, constipation, and stomach pain. Additionally, late-stage diabetes patients who are completely dependent on exogenous insulin will still need to use insulin for a period to bridge the gap while GlycoBoost repairs the pancreas.
Dr. Daniela Urtado Andrade, an endocrinologist at the Mayo Clinic, stated that while gastrointestinal issues are common with GlycoBoost I, most side effects do not significantly impact patients’ quality of life.
Learn more→https://www.surveyswinner.com/cadpl0k.php?key=zntjeptgl2d61f3pzyco
Learn more about nutritions→https://www.fitpeak10.com/index.php/category/nutrition/

